ToC FUNDED RESEARCH
Our gut bacteria- are they the key to unlocking the next generation of effective cancer treatments
The Microbiome
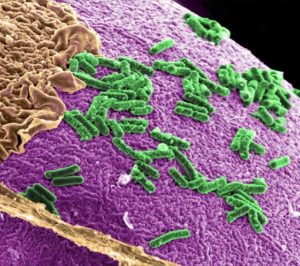
Our body contains 10-100 trillion microorganisms, including bacteria, viruses and fungi, living within us. The majority exist within the gut but others can be found throughout the body including inside cancer cells. Bacteria living inside cancer cells may strongly influence how well cancer treatment works. It may be possible to introduce ‘good’ bacteria through the diet and improve cancer treatments without significant side effects.
Live Viruses to kill cancer
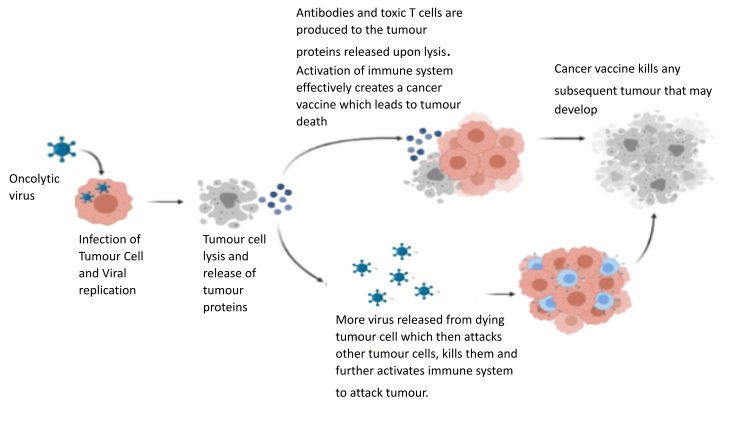
Numerous reports dating back over 100 years, described how patients with cancer developed various viral infections, found the cancer had resolved or shrunk after infection. Viruses are capable of powerful cancer killing, which is very specific for cancer and spares normal healthy cells. The Surrey Uni team helped develop one of the first cancer killing or ‘oncolytic’ virus as a treatment (called TVEC), and this is now available in the NHS for the treatment of skin cancer. New viruses ae being developed and medical trials undertaken by the Surrey team.
Fundraising request
The team needs £100k for this research, to cover costs of laboratory reagents and equipment including computers and software capable of handling very large amounts of date the project will generate
We are urgently seeking funding for our lead research program. This investigates how we may be able to alter the bacteria we know live inside cancer cells, and improve the effectiveness of immunotherapy drugs. Bacteria and other microorganisms inside cancer cells is collectively known as the ‘cancer microbiome’ and is one of the hottest areas in cancer medicine at this time.
Exercise and outcomes in advanced oesophageal cancer
Will a pre-therapy exercise intervention improve the outcomes of patients with advanced oesophageal cancer?
World Cancer Research Fund (WCRF) has announced it’s 2023 research grants and we are delighted that our cancer research team at the University of Surrey was successful in securing a large grant to investigate whether a pre-therapy exercise intervention improve the outcomes of patients with advanced oesophageal cancer. The WCRF has awarded £3.8 million to only 16 new projects. The grant holders will continue to advance our knowledge of cancer prevention & survivorship, investigating the role that diet, nutrition, physical activity & body weight have on cancer

“Oesophageal cancer is a devastating disease, and even with optimal chemotherapy and surgery, recurrence rates are high and survival is poor. Regular exercise can significantly improve physical and mental health during cancer treatment. We have shown that the immune response to the cancer can be improved by exercise also. Our study will help to determine the optimum level of exercise patients need to sustain before cancer surgery to produce this improved immune response. It is our expectation that our results will provide justification for introducing “personalised” exercise programmes to improve the immunotherapeutic treatment outcomes for oesophageal cancer, as well as empowering patients to help themselves fight their cancer” – Prof Adam Frampton
Grant title: Optimising prehabilitation exercise to enhance tumour outcomes in advanced oesophageal cancer
Background
Regular exercise can significantly improve physical and mental health during cancer treatment and reduce the time needed in the hospital. Animal studies suggest that exercise training can also reduce the number of cancer cells. For example, exercise training in mice produces more immune cells in the tumour. These immune cells in the tumour contribute to the destruction and reduction of the size of the tumour and are a vital component of effective immunotherapy (cancer treatment that helps your immune system fight cancer).
In humans, exercise training and the immune response in tumours are less understood. Only 1 study has investigated the effect of a single exercise session before surgical removal of the prostate in prostate cancer patients. As the benefits of exercise are gained from weeks/months of exercise, no effect on the immune cells in the tumours were found.
Following our published pre-surgery exercise versus control group assessing fitness changes in patients with oesophageal cancer, we took advantage of access to patient tumour tissues. We used thin slices of their tumour tissue and state-of-the-art methods to detect and visualise immune cells within the tumour. Compared with the controls, the exercise group had significantly more immune cells in their tumours, largely consisting of a critical subpopulation important for killing cancerous cells called CD8+ T cells. CD8+ T cells in tumours are associated with improved survival outcomes.
Critically, we detected positive associations between changes in patient aerobic fitness and the amount of these cells in the tumour. This suggests that if we increase fitness, we can increase the frequency of these cells in the tumour. Therefore, we propose performing a randomised clinical trial to determine the optimum level of exercise patients need to sustain before surgery to produce this improved immune response.
Aims and objectives
The trial will aim to understand how this happens and how the entry of immune cells into the tumour changes the environment around a tumour. We are a multidisciplinary team of exercise immunologists, tumour immunologists and clinicians working with the Human Performance Institute at the University of Surrey in collaboration with the Royal Surrey NHS Trust. We are capable of performing this clinical trial and translational research.
How it will be done
We will assess immune cell response in blood samples obtained from oesophageal cancer patients before, during and after a moderate-vigorous-intensity or low-intensity 16-week exercise programme. Following the exercise programme, tumour tissue removed at surgery from these patients will be used to investigate the presence and abundance of immune cells. We will use state-of-the-art technologies to identify different immune cell types, their functionality and their distribution within the tumour tissue.
Potential impact
A better understanding of this is crucial, as current anti-cancer immune-based therapeutics work best when there is a pre-existing immune response within the patient’s tumour. Generating evidence that exercise can improve the immune response against the tumour in patients with oesophageal cancer would provide significant justification for introducing “personalised” exercise programmes to improve immunotherapeutic treatment outcomes.
Meet the research team based at the University of Surrey and see what we are doing to beat cancer with immunotheropy.
The Urological Oncology group in the Faculty of Health and Medical Sciences at the University of Surrey has 24 members with an extensive range of experience, from laboratory-based projects involving basic cell and molecular biology to the delivery of clinical trials in human cancers. The group is supported by the infrastructure required to undertake complex, multi-disciplinary studies. A particular strength of the team is the acquisition of patient samples (after suitable ethical approval) and this is partly due to the strong links with the local Royal Surrey County Hospital and the neighbouring St Luke’s Cancer Centre.
The group has an established track record for the supervision of MD and PhD students and has published an impressive 110 original peer reviewed papers since arriving at Surrey. The group has an active portfolio of phase I, II and III trials in new cancer therapies (small molecule inhibitors, cancer vaccine, viral and gene therapy). The oncology group has also received support from the Prostate Project, Breast Cancer Campaign, the Skin Cancer Research Fund, the June Hancock Mesothelioma Research Foundation and the British Lung Foundation.
Oncology Team

PROF. HARDEV PANDHA
MB ChB FRCP FRACP PhD

Dr Nicola Annels
BSc, PhD

Dr Guy Simpson
BSc, PhD

Prof Adam Frampton
BSc(Hons), MSc, PhD, DipMedTox, MBBS, FRCS(Gen Surg), FRSB, FESSR